Background
Information - Section II
Section I: The Arctic
Section II: Earth systems, Processes ang Geoscience
Section III: Climate and Climate Change Research
Section IV: Other
Download:
 PDF: 880 K
PDF: 880 K
 Word: 316 K
Word: 316 K
Background Section II: Earth System Science
What are Earth’s Systems?
The Earth is comprised of various systems, such as, the atmosphere (air),
biosphere (plants and animals, including humans), geosphere (land) and
hydrosphere (water). Like the human body, which has many systems (circulatory,
digestive, respiratory, reproductive, nervous, etc.) it is interactions
between the systems that creates and maintains a stable environment for
life to exist. The environment exists, as we know it today, because of
the processes and interactions happening between the air, plants/animals,
land and waters.
The following concepts about Earth Systems are reprinted from the National
Science Education Standards:
Atmosphere
- Global patterns of atmospheric movement influence local weather. Oceans
have a major effect on climate, because water in the oceans holds a
large amount of heat.
- The atmosphere is a mixture of nitrogen, oxygen, and trace gases that
include water vapor. The atmosphere has different properties at different
elevations.
- Clouds, formed by the condensation of water vapor, affect weather
and climate.
The atmosphere is the mixture of gases, listed below, and water vapor that
surround the earth.
Nitrogen 78%
Oxygen 21%
Argon 0.94%
Carbon Dioxide 0.04%
Helium, Krypton, Neon, Xenon 0.02%
Biosphere
Living organisms have played many roles in the earth system, including affecting
the composition of the atmosphere, producing some types of rocks, and contributing
to the weathering of rocks.
Geosphere
- The solid earth is layered with a lithosphere; hot, convecting mantle;
and dense, metallic core. (See illustration below.)
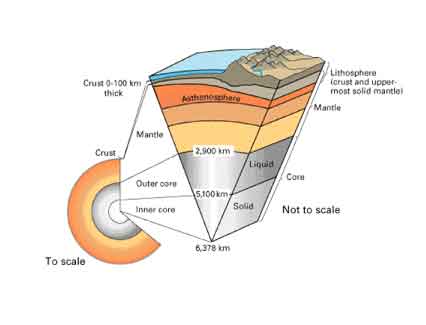
- Cutaway views showing the internal structure of the Earth. Below:
This view drawn to scale demonstrates that the Earth's crust literally
is only skin deep. Below right: A view not drawn to scale to show the
Earth's three main layers (crust, mantle, and core) in more detail.
Illustration and description from the U.S. Geological Survey. http://pubs.usgs.gov/publications/text/inside.html.
- Lithospheric plates on the scales of continents and oceans constantly
move at rates of centimeters per year in response to movements in the
mantle. Major geological events, such as earthquakes, volcanic eruptions,
and mountain building, result from these plate motions.
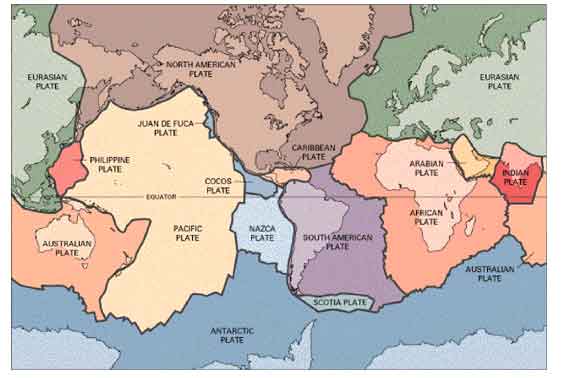
Illustration by U.S. Geological Survey. http://pubs.usgs.gov/publications/text/historical.html
- Landforms are the result of a combination of constructive and destructive
forces. Constructive forces include crustal deformation, volcanic eruption,
and deposition of sediment, while destructive forces include weathering
and erosion.
- Some changes in the solid earth can be described as the "rock
cycle." Old rocks at the earth's surface weather, forming sediments
that are buried, then compacted, heated, and often recrystallized into
new rock. Eventually, those new rocks may be brought to the surface
by the forces that drive plate motions, and the rock cycle continues.
Hydrosphere
- Water, which covers the majority of the earth's surface, circulates
through the crust, oceans, and atmosphere in what is known as the "water
cycle." Water evaporates from the earth's surface, rises and cools
as it moves to higher elevations, condenses as rain or snow, and falls
to the surface where it collects in lakes, oceans, soil, and in rocks
underground.
- Water is a solvent. As it passes through the water cycle it dissolves
minerals and gases and carries them to the oceans.
"What are Earth’s Processes?"
As with our circulatory system, which stores and transports water, oxygen
and nutrients to the various part of the body, the earth systems hold
and transport materials as well. Materials are stored in reservoirs
and they move via pathways/processes to and from other systems.
There are many cycles or processes that occur on earth everyday. These
processes are made possible because of the addition of energy from the
sun.
Energy Cycle
The sun is the major source of energy for phenomena on the earth's surface,
such as growth of plants, winds, ocean currents, and the water cycle.
Seasons result from variations in the amount of the sun's energy hitting
the surface, due to the tilt of the earth's rotation on its axis and
the length of the day. In addition, to the amount of energy available
is the type of energy that is available.
There are many forms of energy
(E) (Download
Energy Cycle.pdf) For instance, energy can be in the form of: light
or heat (like that of the sun), sound, electricity, motion/work, chemical
(i.e. photosynthesis or muscular energy) or nuclear energy. Various
forms of energy change into each other constantly. Energy is also the
reason that materials, such as water, can change states and move through
the water cycle. Using the diagrams of various cycles (water, carbon,
etc.) identify the processes, for instance evaporation, where materials
gain energy and move from one area to another.
Hydrological Cycle
The hydrological (water) cycle provides a model for understanding the
global plumbing system. Water spends time in the ocean, in the air,
on the surface, and under the surface as groundwater. The hydrological
cycle is a closed system because water is neither created nor destroyed
on a large scale. Water exists as solid, liquid and gas phases that
are interchangeable at temperatures found on earth. The hydrological
cycle describes the movement of water as it passes through these phases.
Water links the atmosphere, oceans and land through energy and matter
exchanges as it evaporates, precipitates and flows.
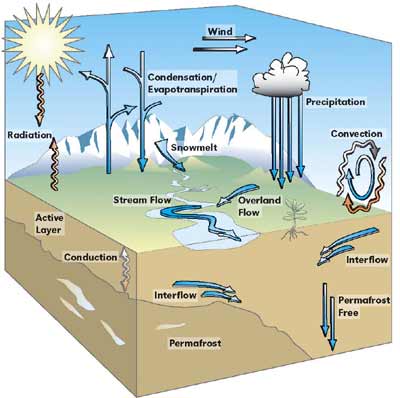
Illustration by Russ Mitchell for Arctic Research
Consortium of U.S.
Carbon Cycle
All life is based on the element carbon. Carbon is the major chemical
constituent of most organic matter, from fossil fuels to the complex
molecules (DNA and RNA) that control genetic reproduction in organisms.
Carbon is stored on our planet in the following major sinks (1) as organic
molecules in living and dead organisms found in the biosphere; (2) as
the gas carbon dioxide in the atmosphere; (3) as organic matter in soils;
(4) in the lithosphere as fossil fuels and sedimentary rock deposits
such as limestone, dolomite and chalk; and (5) in the oceans as dissolved
atmospheric carbon dioxide and as calcium carbonate shells in marine
organisms.
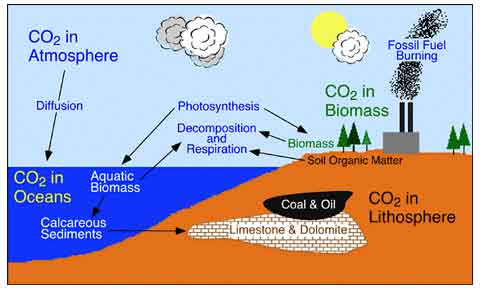
Illustration and carbon description from Department
of Geography, Okanagan University College.
Ecosystems gain most of their carbon dioxide from the atmosphere. A
number of autotrophic organisms have specialized mechanisms that allow
for absorption of this gas into their cells. With the addition of water
and energy from solar radiation, these organisms use photosynthesis
to chemically convert the carbon dioxide to carbon-based sugar molecules.
These molecules can then be chemically modified by these organisms through
the metabolic addition of other elements to produce more complex compounds
like proteins, cellulose, and amino acids. Some of the organic matter
produced in plants is passed down to heterotrophic animals through consumption.
Carbon dioxide enters the waters of the ocean by simple diffusion. Once
dissolved in seawater, the carbon dioxide can remain as is or can be
converted into carbonate (CO3-2) or bicarbonate (HCO3-). Certain forms
of sea life biologically fix bicarbonate with calcium (Ca+2) to produce
calcium carbonate (CaCO3). This substance is used to produce shells
and other body parts by organisms such as coral, clams, oysters, some
protozoa, and some algae. When these organisms die, their shells and
body parts sink to the ocean floor where they accumulate as carbonate-rich
deposits. After long periods of time, these deposits are physically
and chemically altered into sedimentary rocks. Ocean deposits are by
far the biggest sink of carbon on the planet.
Carbon is released from ecosystems as carbon dioxide gas by the process
of respiration. Respiration takes place in both plants and animals and
involves the breakdown of carbon-based organic molecules into carbon
dioxide gas and some other compound by products. The detritus food chain
contains a number of organisms whose primary ecological role is the
decomposition of organic matter into its abiotic components.
For a fun look at the carbon cycle, go to http://library.thinkquest.org/11226/index.htm.
Nutrient Cycle
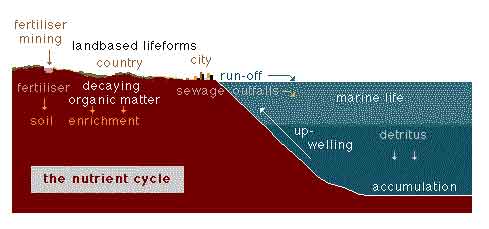
Illustration and caption with permission from Matthias
Tomczak © 2000 M.Tomczak
The natural cycle consists of the recycling of decaying organic matter
into land-based life forms on land and nutrient supply for marine from
upwelling in the ocean.
The development of human civilization introduces the additional elements
of sewage disposal and fertilizer application.
What are Geosciences?
Geosciences are sciences, such as physics, chemistry or geology,
that are applied to the study the earth. Each piece of additional
research continues to add to our understanding of how the earth systems
and processes interact. Combining research from many fields helps to
provide a complete picture to help us build accurate models of the earth.
Accurate models can help us to predict outcomes or consequences of change,
for instance, those changes associated with climate change.
It is becoming very clear to scientists that it will take a global research
effort, combining knowledge from a variety of perspectives, to understand
all the of the variables affecting the climate. Although, it is impossible
to know everything, there are a few basic scientific facts that are
important to know. These basic facts are applied across disciplines
in a variety of studies to better understand the environment.
What are a few things I should know about Chemistry, Physics, and the
Science of Light?
Chemistry
Chemistry is the study of the elements which form all existing substances.
It covers their structure, how they combine to create other substances
and how they react under various conditions. There are four areas of
chemistry: physical, inorganic, organic, and environmental. For more
information about chemistry and the periodic table go to the following
web site: http://pearl1.lanl.gov/periodic/default.htm
. To understand more about molecules, go to the following web site:
http://www.recipnet.indiana.edu/common/common.html
About the Molecule Called “Water”:
One of the most important element is water. “Water is a substance
made up of three atoms: two atoms of hydrogen and one atom of oxygen.
These three atoms are held together by electrical forces, or bonds,
in an arrangement called a molecule. Each hydrogen atom shares its electron
with the oxygen atom creating a bond between the atoms.
Vapor. This form of water has no shape or cohesion. It is created
when water molecules are heated to such a fast and furious movement
that the bonds linking them together break apart. Climate models predict
that warmer temperatures will cause increased evaporation of water into
the air.
Liquid. In this form, each water molecule has stronger, more
numerous bonds with its neighbors. Chilling the bonds tightens the bonds
even further. If climate warms, the volume of the water in the oceans
might increase and sea levels might rise. This volume increase would
be due to two factors. First, if the oceans warm then water expands
as it warms. Second, much water now locked into the polar ice caps would
melt into liquid form.
Ice. This form is cold, hard and rigid because each of its water
molecules has many firm bonds with its neighboring molecules. Today
land and sea ice reflects into space approximately 10 percent of the
sunlight that reaches us, and helps to cool the earth.”
National Science Teachers Association. (1996). Forecasting the Future.
Graphic Communications, Inc.
Physics
Physics is the study of the properties and nature of matter,
the different forms of energy and the ways in which matter and energy
interact in the world around us. To understand physics, you need to
understand what matter is. For more information about physics,
go to the following web site: http://kapili.com/m/modernphys.html
Some Laws of Physics
- Action (A) = Reaction(R)
Every action has an equal and opposite reaction.
- Energy is defined as the capacity to do work. The release of energy
does work and doing work on something adds energy to it. Work and energy
are equivalent.
- Law of Conservation of Energy
The total energy of a system does not change (it is neither created
nor destroyed) it is simply converted to another form
The Science of Light
Light interacts with matter by transmission (including refraction),
absorption or scattering (including reflection). This concept
becomes important in regards to climate change because the amount of
energy/light that is absorbed by the planet not only is available for
use in earth processes (i.e. for evaporation) this energy can also be
trapped within the earth system as heat. Therefore, the amount of energy
reflected back into space and the amount absorbed by the planet have
impacts on the temperature of the earth.
To understand more about the light spectrum, refraction, and
reflection, check out the following web sites:
http://imagers.gsfc.nasa.gov/ems/visible.html
http://www.kapili.com/l/light.html
Section I: The Arctic
Section II: Earth systems, Processes ang Geoscience
Section III: Climate and Climate Change
Research
Section IV: Other
|

